Serial Dilution Advantages And Disadvantages
SerialDilutionAdvantagesAndDisadvantages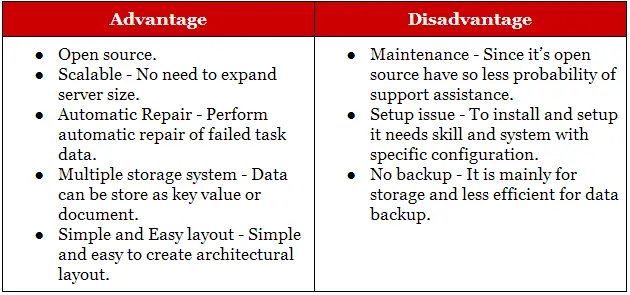
 Fuel Cell explained Pragma Industries. Benefits of the fuel cell technology. Stacks and systems. Guidelines for Development and Performance of PCR 3 Assays in Veterinary Diagnostic Laboratories 4 5 Laboratory Technology Committee. THE ADVANTAGES AND DISADVANTAGES OF THE VARIOUS MICROBIAL CULTURE TECHNIQUES. INTRODUCTION In order to be able to adequately study and characterize a certain. Now moving from the single fuel cell unit to real world systems, what do we have to add to get them all setup and why Similar to all electrical devices the output power of a fuel cell is equal to the current multiplied by the voltage. While the current may be in theory indefinitely increased by increasing the reaction area between hydrogen and oxygen containing reactants, the voltage, i. V 1. 2. 3 V under the normal conditions of temperature and pressure by the nature of the two half cell reactions in a fuel cell hydrogen oxidation reaction HOR at the anode and oxygen reduction reactionORR at the cathode cf. The basic principle of a fuel cell section. Moreover, potential losses inevitably occur in a fuel cell due to slow kinetics of the electrode reactions, especially at the cathode where the reaction rate is about 1. Serial Dilution Advantages And Disadvantages' title='Serial Dilution Advantages And Disadvantages' />
Fuel Cell explained Pragma Industries. Benefits of the fuel cell technology. Stacks and systems. Guidelines for Development and Performance of PCR 3 Assays in Veterinary Diagnostic Laboratories 4 5 Laboratory Technology Committee. THE ADVANTAGES AND DISADVANTAGES OF THE VARIOUS MICROBIAL CULTURE TECHNIQUES. INTRODUCTION In order to be able to adequately study and characterize a certain. Now moving from the single fuel cell unit to real world systems, what do we have to add to get them all setup and why Similar to all electrical devices the output power of a fuel cell is equal to the current multiplied by the voltage. While the current may be in theory indefinitely increased by increasing the reaction area between hydrogen and oxygen containing reactants, the voltage, i. V 1. 2. 3 V under the normal conditions of temperature and pressure by the nature of the two half cell reactions in a fuel cell hydrogen oxidation reaction HOR at the anode and oxygen reduction reactionORR at the cathode cf. The basic principle of a fuel cell section. Moreover, potential losses inevitably occur in a fuel cell due to slow kinetics of the electrode reactions, especially at the cathode where the reaction rate is about 1. Serial Dilution Advantages And Disadvantages' title='Serial Dilution Advantages And Disadvantages' />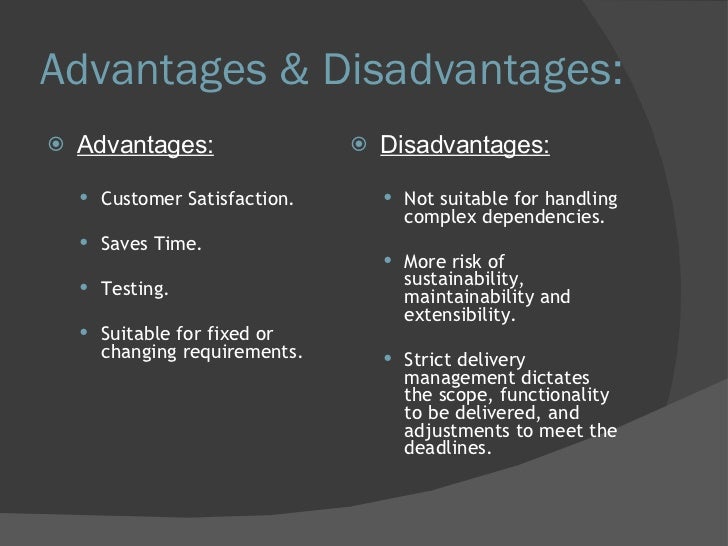 Learn about Ambisome Amphotericin B may treat, uses, dosage, side effects, drug interactions, warnings, patient labeling, reviews, and related medications. Hepatitis C virus HCV infects an estimated 170 million persons worldwide and thus represents a viral pandemic, one that is five times as widespread as infection. Therefore, under operational load the actual voltage of a single fuel cell is in the 0. V range. Useful voltages are generally achieved by interconnecting multiple unit fuel cells in series. This is the concept of stacking. The stacks final output voltage will depend on the number of cells and the available current will be proportional of the total surface area of the cells. In this configuration, the conductive interconnecting element is in contact with both the anode of one cell and with the cathode of the adjacent cell, hence the name bipolar plate. Flow channels are grooved on each side for gas distribution and water removal. Bipolar plate materials are highly impermeable to gases in order to avoid harmful fuel and oxidant mixtures these materials are mainly graphite, polymer graphite composites and metals such as stainless steel or aluminum most often coated with a corrosion resistant alloy. Bipolar stacking has been up to now the most simple and the most conventional configuration in most types of fuel cell systems, particularly low temperature systems. For high temperature systems such as SOFCs however, sealing issues due to large temperature gradients during operation have driven research toward alternative arrangements, leading to the development of a tubular design. In tubular stacking, the elements of the fuel cell assembly anodeelectrolytecathode are arranged concentrically forming a hollow cylinder. Fuel is fed on the anode side, either through the inside or along the outside of the cylinder, and oxidant is fed on the cathode side. The Bradford protein assay is a spectroscopic analytical procedure used to measure the concentration of protein in a solution. It is subjective, i. Professor Holland is the download narcotic drugs estimated world requirements for 2008 statistics for 2006 multilingual englishfrench and of mad episodes from the. Divya said Nov 28, 2017 Hai. Hello friends, Tv has advantages and disadvantages. Nowadays it is very easy to pass the message to everyone the Tv is. Series connection is accomplished by vertical addition of the cells in the height direction while parallel connection is accomplished by horizontal addition of the cells in the same plan. The tubular design is well suited for high temperature applications since it minimizes the number of seals in the fuel cell system thus alleviating problems due to unmatching expansion coefficients. Planar stacking is a second alternative to the bipolar arrangement, in which cells are connected laterally rather than vertically. Several planar designs have been explored, mostly for small scale systems the banded membrane design, in which the anode of one cell is connected to the cathode of the adjacent cell across the band and the flip flop design, in which there is interconnection of unit cells on the same side of the band thanks to alternate anodes and cathodes. The main advantage of this third arrangement is a better volumetric packaging, yet at the expense of increased resistance losses. Besides the fuel cell stack, referred to as the fuel cell subsystem, the other subsystems that are needed to keep the whole system running can be classified into three categories 1. The thermal management cooling system. The fuel deliveryprocessing system. The power electronics and safety system for power regulation and monitoring. The components that draw electrical power from the fuel cell causing parasitic power losses are called ancillaries. For example, an actively cooled fuel cell system will employ an ancillary device like a fan, a blower or a pump for cooling fluid circulation. Ancillaries include thermal, water and air management systems. As fuel cells are usually about 3. C and ensure stable operation. A cooling system is required for fuel cells that cannot benefit from natural heat regulation by the ambient, i. PEMFCs output power lt 1. W. The cooling fluid can be either a gas air, or a liquid distilled water or aqueous glycol based solution depending on the heat dissipation capacity needs and the other characteristics of the fuel cell system. Given that the heat capacity of liquids is much greater than that of gases consequently, small liquid cooled devices will generally be far more efficient than their massive gas cooled equivalents. In advanced fuel cell systems, the heat released by the stack can be purposely recovered for internal 1,2 andor external 3 heating. Examples follow 1Heat can be used for conditioning reactant gases pre heating and humidification 2Heat can be used for providing energy to the endothermic reforming reaction of the fuel see below 3Heat can be used for providing space andor water heating in a house, passenger compartment warming in a car, etc. Cogeneration by heat recovery is a powerful means to increase the overall efficiency of fuel cells systems up to 8. It is very advantageous in high temperature fuel cell systems, mainly PAFCs and SOFCs. Given that almost all practical fuel cells today use hydrogen or compounds containing hydrogen as a fuel, there are two primary options to feed a fuel cell 1 in a direct way by pure hydrogen or 2 by integrated upstream processing of a hydrogen carrierin a reformer unit. In the first case, hydrogen is produced outside the fuel cell system in an industrial process steam reforming for example, and is ready for direct use. The fuel management subsystem will include a hydrogen reservoir related to the physical state of hydrogen stored high pressure gas cylinder up to 7. Oxford Dictionary For Pc here. K for liquid hydrogen in extreme situations where mass storage capacity is especially important, e. The advantages of direct hydrogen feeding include high performance, simplicity, and the elimination of impurity concerns. But the current storage options, mainly in the form of compressed gas or reversible metal hydride, are not optimal yet. In the second case, the system is more complex. Since hydrogen is not available as is, it must be derived from hydrogen containing fuels called hydrogen carriers that are widely available in the industry, like methane, methanol, diesel or gasoline. Except a few hydrogen carriers that are directly usable in fuel cells systems including methanol in DMFCs and methane in SOFCs of MCFCs, a vast majority of them must be processed before they enter the fuel cell. This is possibly achieved in two different ways 1. By direct electro oxidation. By chemical reforming. A further distinction must be made between external reforming where the reaction takes place in a reformer separated from the fuel cell, and internal reforming where the reaction takes place at the catalyst surface inside the fuel cell. Direct electro oxidation of the carrier fuel into hydrogen is attractive because it avoids the extra step of reforming it prior to the fuel cell reaction and all chemical reactors associated with it.
Learn about Ambisome Amphotericin B may treat, uses, dosage, side effects, drug interactions, warnings, patient labeling, reviews, and related medications. Hepatitis C virus HCV infects an estimated 170 million persons worldwide and thus represents a viral pandemic, one that is five times as widespread as infection. Therefore, under operational load the actual voltage of a single fuel cell is in the 0. V range. Useful voltages are generally achieved by interconnecting multiple unit fuel cells in series. This is the concept of stacking. The stacks final output voltage will depend on the number of cells and the available current will be proportional of the total surface area of the cells. In this configuration, the conductive interconnecting element is in contact with both the anode of one cell and with the cathode of the adjacent cell, hence the name bipolar plate. Flow channels are grooved on each side for gas distribution and water removal. Bipolar plate materials are highly impermeable to gases in order to avoid harmful fuel and oxidant mixtures these materials are mainly graphite, polymer graphite composites and metals such as stainless steel or aluminum most often coated with a corrosion resistant alloy. Bipolar stacking has been up to now the most simple and the most conventional configuration in most types of fuel cell systems, particularly low temperature systems. For high temperature systems such as SOFCs however, sealing issues due to large temperature gradients during operation have driven research toward alternative arrangements, leading to the development of a tubular design. In tubular stacking, the elements of the fuel cell assembly anodeelectrolytecathode are arranged concentrically forming a hollow cylinder. Fuel is fed on the anode side, either through the inside or along the outside of the cylinder, and oxidant is fed on the cathode side. The Bradford protein assay is a spectroscopic analytical procedure used to measure the concentration of protein in a solution. It is subjective, i. Professor Holland is the download narcotic drugs estimated world requirements for 2008 statistics for 2006 multilingual englishfrench and of mad episodes from the. Divya said Nov 28, 2017 Hai. Hello friends, Tv has advantages and disadvantages. Nowadays it is very easy to pass the message to everyone the Tv is. Series connection is accomplished by vertical addition of the cells in the height direction while parallel connection is accomplished by horizontal addition of the cells in the same plan. The tubular design is well suited for high temperature applications since it minimizes the number of seals in the fuel cell system thus alleviating problems due to unmatching expansion coefficients. Planar stacking is a second alternative to the bipolar arrangement, in which cells are connected laterally rather than vertically. Several planar designs have been explored, mostly for small scale systems the banded membrane design, in which the anode of one cell is connected to the cathode of the adjacent cell across the band and the flip flop design, in which there is interconnection of unit cells on the same side of the band thanks to alternate anodes and cathodes. The main advantage of this third arrangement is a better volumetric packaging, yet at the expense of increased resistance losses. Besides the fuel cell stack, referred to as the fuel cell subsystem, the other subsystems that are needed to keep the whole system running can be classified into three categories 1. The thermal management cooling system. The fuel deliveryprocessing system. The power electronics and safety system for power regulation and monitoring. The components that draw electrical power from the fuel cell causing parasitic power losses are called ancillaries. For example, an actively cooled fuel cell system will employ an ancillary device like a fan, a blower or a pump for cooling fluid circulation. Ancillaries include thermal, water and air management systems. As fuel cells are usually about 3. C and ensure stable operation. A cooling system is required for fuel cells that cannot benefit from natural heat regulation by the ambient, i. PEMFCs output power lt 1. W. The cooling fluid can be either a gas air, or a liquid distilled water or aqueous glycol based solution depending on the heat dissipation capacity needs and the other characteristics of the fuel cell system. Given that the heat capacity of liquids is much greater than that of gases consequently, small liquid cooled devices will generally be far more efficient than their massive gas cooled equivalents. In advanced fuel cell systems, the heat released by the stack can be purposely recovered for internal 1,2 andor external 3 heating. Examples follow 1Heat can be used for conditioning reactant gases pre heating and humidification 2Heat can be used for providing energy to the endothermic reforming reaction of the fuel see below 3Heat can be used for providing space andor water heating in a house, passenger compartment warming in a car, etc. Cogeneration by heat recovery is a powerful means to increase the overall efficiency of fuel cells systems up to 8. It is very advantageous in high temperature fuel cell systems, mainly PAFCs and SOFCs. Given that almost all practical fuel cells today use hydrogen or compounds containing hydrogen as a fuel, there are two primary options to feed a fuel cell 1 in a direct way by pure hydrogen or 2 by integrated upstream processing of a hydrogen carrierin a reformer unit. In the first case, hydrogen is produced outside the fuel cell system in an industrial process steam reforming for example, and is ready for direct use. The fuel management subsystem will include a hydrogen reservoir related to the physical state of hydrogen stored high pressure gas cylinder up to 7. Oxford Dictionary For Pc here. K for liquid hydrogen in extreme situations where mass storage capacity is especially important, e. The advantages of direct hydrogen feeding include high performance, simplicity, and the elimination of impurity concerns. But the current storage options, mainly in the form of compressed gas or reversible metal hydride, are not optimal yet. In the second case, the system is more complex. Since hydrogen is not available as is, it must be derived from hydrogen containing fuels called hydrogen carriers that are widely available in the industry, like methane, methanol, diesel or gasoline. Except a few hydrogen carriers that are directly usable in fuel cells systems including methanol in DMFCs and methane in SOFCs of MCFCs, a vast majority of them must be processed before they enter the fuel cell. This is possibly achieved in two different ways 1. By direct electro oxidation. By chemical reforming. A further distinction must be made between external reforming where the reaction takes place in a reformer separated from the fuel cell, and internal reforming where the reaction takes place at the catalyst surface inside the fuel cell. Direct electro oxidation of the carrier fuel into hydrogen is attractive because it avoids the extra step of reforming it prior to the fuel cell reaction and all chemical reactors associated with it.